By Anna Iizuka
Reading time: ~25 minutes
Why Japanese Fermentation Is the Biggest Opportunity in Nutraceuticals Right Now
Here is a number that shouldget your attention: the global fermented ingredients market is projected to reach 102.6 billion USD by 2035, growing at a CAGR of 6.3%. The nutraceutical ingredients market alone is expected to hit 191 billion USD by 2034. And at thevery center of this growth sits a technology that Japan has been perfecting forover a thousand years.
Fermentation is not new. What is new is the scientific understanding of why it works, the clinical evidence proving it, and the consumer demand pulling it forward. Gut health, postbiotics, clean-label formulations, biotransformation of plant compounds into more bioavailable forms - these are not buzzwords anymore. They are product development man dates for any supplement brand that wants to stay relevant in 2026 and beyond.
Yet here is the paradox: while Western supplement brands chase the latest probiotic strains and precision fermentation technologies, Japan's traditional fermentation systems -koji molds, natto bacteria, lactic acid fermentation of vegetables and grains -remain largely unexplored by international formulators. This is not because these systems lack science. It is because the science, the clinical data, and the supply chains have been locked inside Japan's domestic market.
This article changes that.
What follows is aformulator-level deep dive into Japanese fermentation science: the mechanisms, the bioactive compounds, the clinical evidence, and the practical applications for supplement and functional food development. Whether you are an R&D specialist evaluating new ingredients, a brand owner looking for differentiation, or aprocurement manager sourcing premium raw materials, this guide will give youthe technical foundation you need to make informed decisions.
We will cover three pillars of Japanese fermentation - koji enzyme systems, natto biotransformation, and miso-derived postbiotics - and then move into practical formulation guidance with real ingredients you can source today.
Let us begin.
Koji: The Master Enzyme System Behind Japanese Fermentation
If there is a single organism that defines Japanese fermentation, it is Aspergillus oryzae -the koji mold. Designated Japan's "national fungus" (kokkin) in 2006 by the Brewing Society of Japan, koji is the enzymatic engine behind sake,miso, soy sauce, mirin, and dozens of traditional fermented foods. But its relevance extends far beyond the kitchen.
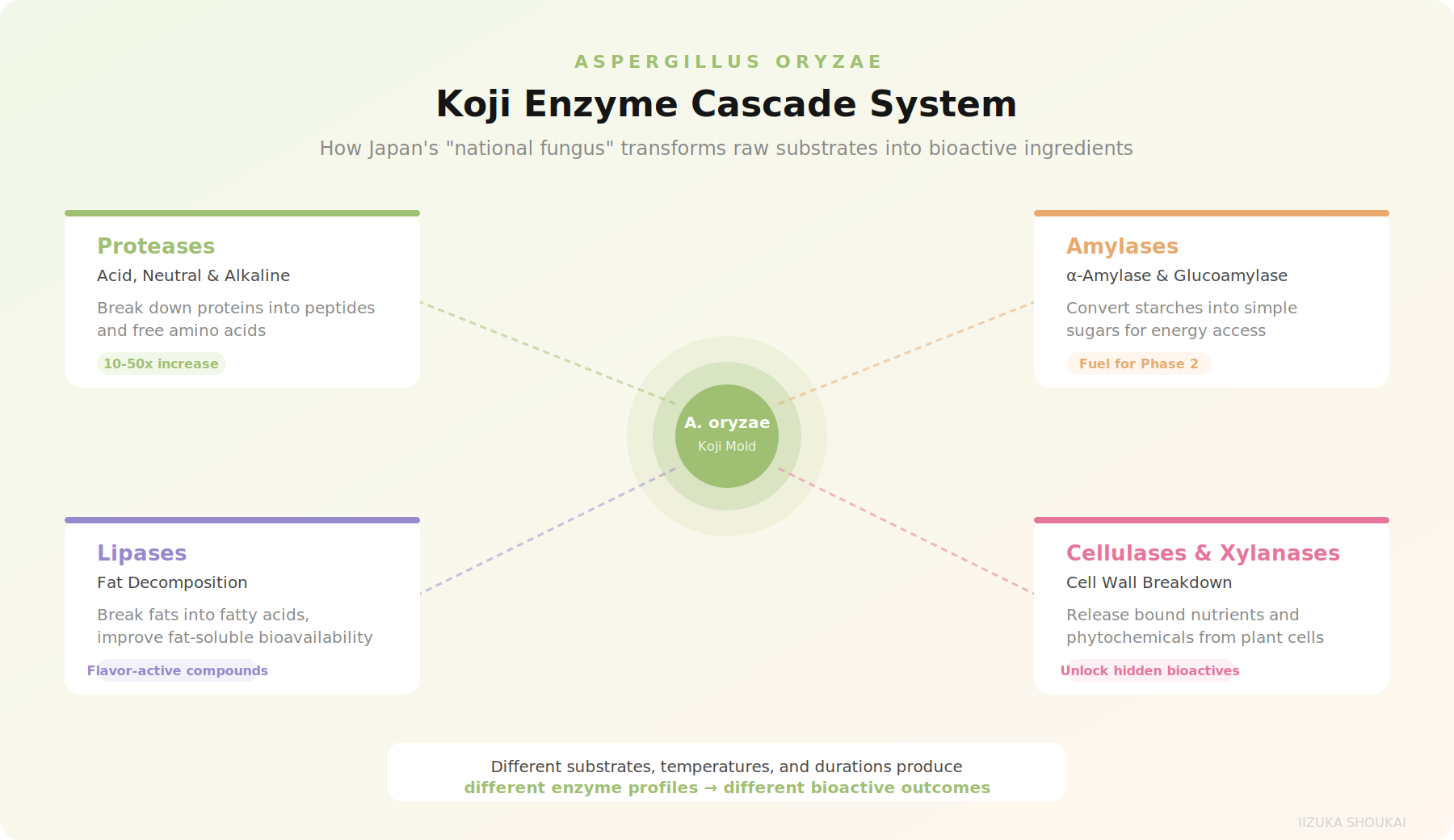
How Koji Works: An Enzyme Factory on a Microscopic Scale
Koji fermentation is fundamentally different from the lactic acid or ethanol fermentation familiar to most Western formulators. Instead of producing a single metabolite, A.oryzae secretes a complex cocktail of enzymes that systematically breakdown the substrate it grows on. The result is not just preservation or flavordevelopment, but a genuine biotransformation of the raw material into something with a fundamentally different nutritional and bioactive profile.
The primary enzyme systemsinclude:
• Proteases (acid, neutral, and alkaline): These break down proteins into peptides and free amino acids. In a soy substrate, koji proteases can increase free amino acid content by 10-50x depending on fermentation conditions. This is not just about digestibility - many of the resulting peptides have independent bioactivity, including ACE-inhibitory effects relevant to cardiovascular health.
• Amylases (alpha-amylase, glucoamylase): These convert starches into simple sugars, making carbohydrate energy more accessible and serving as fuel for secondary fermentation stages. Alpha-amylase from koji is particularly heat-stable, which matters for processing.
• Lipases: These break down fats into fatty acids and glycerol, improving the bioavailability of fat-soluble compounds and generating flavor-active compounds.
• Cellulases and xylanases: These break down plant cell walls,releasing bound nutrients and phytochemicals that would otherwise pass through the GI tract unabsorbed.
What makes koji particularly interesting for nutraceutical applications is that this enzyme production is not random. Different substrates, temperatures, humidity levels, and fermentation durations produce different enzyme profiles. Japanese manufacturers have spent decades - in some cases, centuries - optimizing these parameters for specific outcomes.
Koji-Derived Bioactive Compounds
Beyond the enzymes themselves, koji fermentation generates a range of bioactive metabolites:
• Ergothioneine: A potent antioxidant amino acid that accumulates in mitochondria.Koji-fermented products are among the richest dietary sources, and ergothioneine is increasingly recognized for its neuroprotective and anti-aging properties.
• Pyroglutamyl peptides: Unique peptides formed during koji fermentation of soy proteins.Research suggests anti-inflammatory and immunomodulatory activity.
• Polyamines (spermidine, spermine): Koji-fermented soy products containelevated levels of spermidine, a compound with growing evidence for autophagyinduction and longevity support.
• Isoflavone aglycones: Koji enzymes convert soy isoflavone glycosides into their aglyconeforms (genistein, daidzein), which are 2-3x more bioavailable than the glycoside precursors.
The practical takeaway for formulators: a koji-fermented ingredient is not simply a "natural"version of its unfermented counterpart. It is a different ingredient with different bioactivity, different bioavailability, and different clinical potential.
The Ergothioneine Opportunity
Among koji-derived compounds, ergothioneine deserves special attention from formulators. Thissulfur-containing amino acid is not synthesized by humans, yet our bodies maintain a dedicated transporter for it (OCTN1/SLC22A4), suggesting strong evolutionary selection for its uptake. Ergothioneine accumulates preferentially in tissues with high oxidative stress exposure: the liver, kidneys, bonemarrow, lens of the eye, and seminal fluid.
Recent research has linked ergothioneine intake to reduced risk of cardiovascular mortality, cognitivedecline, and frailty in aging populations. A 2020 study in the journal Free Radical Biology and Medicine found that low plasma ergothioneine levels correlated with increased risk of cardiovascular disease events. The European Food Safety Authority has approved ergothioneine as a novel food ingredient, opening regulatory pathways for supplementation.
Koji-fermented products -particularly those using rice or soy substrates fermented with optimized A.oryzae strains - represent one of the most concentrated dietary sources of ergothioneine. For formulators looking to enter the longevity and healthy aging supplement space, this is a compelling differentiator: a naturally occurring compound with clinical relevance, delivered through a traditional fermentation process that consumers already associate with health and quality.
Understanding Biotransformation: Why Fermented Ingredients Are Not Just 'Pre-Digested'
A common misconception in the supplement industry is that fermentation simply "pre-digests" raw materials, making nutrients easier to absorb. While improved digestibility is certainly one outcome, the reality is far more complex and far more valuable from a formulation perspective.
Biotransformation through fermentation creates entirely new compounds that did not exist in the starting material. When koji mold ferments a multi-botanical substrate, the enzymatic cascade does not just break bonds - it forms new ones. Peptide fragments recombine. Sugar residues are cleaved from phenolic compounds, changing their biological activity. Amino acids undergo transamination, decarboxylation, and condensation reactions that produce novel metabolites.
This is why a fermented botanical extract is genuinely different from an unfermented extract that has been treated with commercial enzymes. The fermentation environment - the interplay of multiple enzyme systems, the pH gradients, the shifting oxygen availability,the secondary metabolites that inhibit or promote specific reaction pathways -creates a level of chemical complexity that cannot be replicated by adding purified enzymes to a substrate in a reactor.
For product claims and marketing, this distinction matters. A fermented ingredient is not just"better absorbed." It is a new ingredient with unique bioactive compounds, supported by its own clinical evidence, and worthy of its own branded positioning.
Case Study: BIOZYME - 40+ Botanicals, Double Fermentation,Triple Benefit
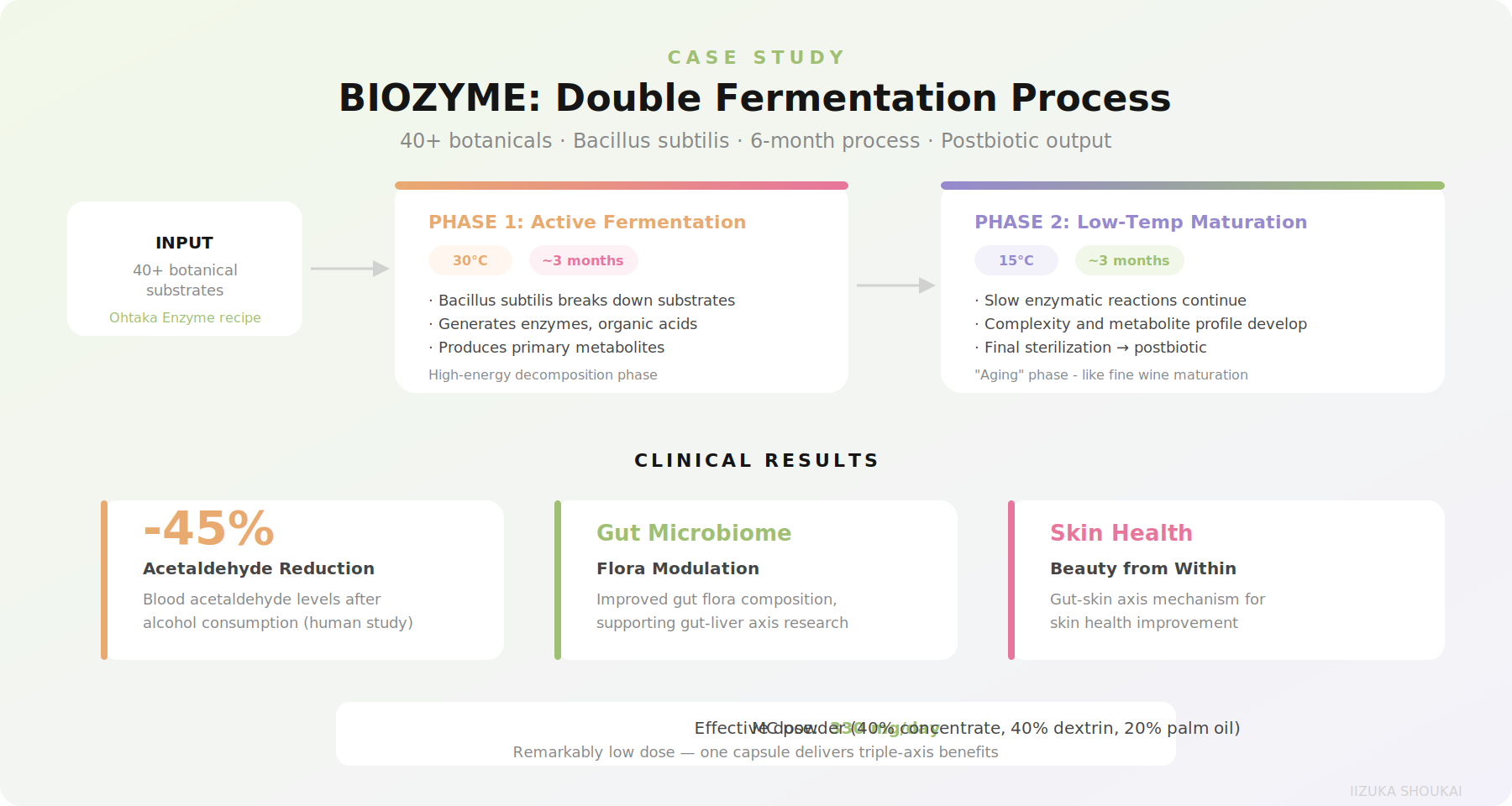
To see koji-style multi-stage fermentation in action at commercial scale, consider BIOZYME - a fermented vegetable extract developed by Japan Bio Science Laboratory using Ohtaka Enzyme's 40+ botanical substrate. BIOZYME uses a proprietary Bacillussp. strain (closely related to the fermentation ecology of traditional Japanese processes) and a distinctive 6-month double fermentation protocol.
The process works in two distinct phases:
• Phase 1 (30 degrees C, ~3 months): Active fermentation where the Bacillusstrain breaks down the botanical substrate, generating enzymes, organic acids,and metabolites. This is the high-energy phase where most biotransformation occurs.
• Phase 2 (15 degrees C, ~3 months): Low-temperature maturation where enzymatic reactions continue slowly, complexity develops, and the metabolite profile stabilizes. Think of this as the "aging" phase - similar inconcept to how sake or miso develops depth through extended low-temperature fermentation.
The resulting extract is a postbiotic - the cells are sterilized, so the product's benefits come from the metabolites and transformed compounds, not from live organisms. This is acritical formulation advantage: no cold chain requirement, long shelf life, and consistent potency.
Clinical data on BIOZYME reveals a multi-benefit profile that would be difficult to achieve with single-compound ingredients:
• Acetaldehyde reduction (~45%): In human studies, BIOZYME supplementation before alcohol consumption significantly reduced blood acetaldehyde levels. Acetaldehyde is the primary toxic metabolite of alcohol and a major driver of hangover symptoms and long-term liver stress.
• Gut microbiome modulation: Improvements in gut flora composition,supporting the growing "gut-liver axis" research area.
• Skin health improvements: Via the gut-skin axis mechanism,supporting the beauty-from-within category.
From a formulation perspective, BIOZYME works as a 330 mg/day dose in MC powder form. That is are markably low dose compared to many botanical extracts - a reflection of the concentration achieved through extended fermentation.
BIOZYME's multi-benefit profile (gut + liver + beauty) allows a singleingredient to support multiple product claims, simplifying formulation andreducing capsule count. Compare this to standard botanical extracts requiring 1000 mg doses.
Natto Fermentation: Nattokinase and the Bioactives You Have Not Heard About
If koji is Japan's master enzyme system, natto fermentation is its cardiovascular health power house. Bacillussubtilis var. natto - the bacterium responsible for fermenting soybeans into natto - produces nattokinase, one of the most clinically validated natural fibrinolytic enzymes known. But nattokinase is only part of the story.

Nattokinase: What Formulators Actually Need to Know
Nattokinase (NK) is a serine protease with direct fibrinolytic activity - it breaks down fibrin, the protein that forms the structural basis of blood clots. Unlike pharmaceutical thrombolytics, nattokinase also enhances the body's own fibrinolytic system by activating pro-urokinase and increasing tissue plasminogen activator (t-PA) levels.
The standard commercial specification is 2,000 FU (fibrinolytic units) per 100 mg, though higher-potency extracts are available. Key formulation considerations:
• Dosage: Most clinical studies use 2,000-5,000 FU/day. The Japan Nattokinase Association recommends 2,000 FU/day for general cardiovascular maintenance.
• Vitamin K2 content: Whole natto contains significant vitamin K2 (MK-7), which can interact with anticoagulant medications. Purified nattokinase extracts are typically vitamin K-removed, but formulators must verify this with suppliers and declareit clearly on product labels.
• Stability: Nattokinase is relatively heat-sensitive. Processing temperatures above 60 degrees C will significantly reduce activity. Enteric coating oracid-resistant capsules improve oral bioavailability.
• Allergen considerations: Nattokinase is derived from soy fermentation. While the fermentation process significantly reduces allergenic protein content, soy declaration is required in most markets. Some manufacturers offer non-soy nattokinase produced via recombinant Bacillus expression, but these products lack the full spectrum of natto-derived bioactives.
Beyond Nattokinase: The Full Natto Bioactive Spectrum
Natto fermentation produces a complex matrix of bioactives that are often overlooked in the rush to isolate nattokinase:
• Dipicolinic acid (DPA): A compound produced by Bacillus subtilis spores with demonstrated antibacterial, antifungal, and potential anti-tumor properties. DPA also chelates calcium, which may influence bone metabolism.
• Poly-gamma-glutamic acid (PGA): The sticky, stringy component of natto. PGA has been studied for moisture retention, calcium absorption enhancement,and as a drug delivery vehicle.
• Menaquinone-7 (Vitamin K2-MK7): Natto is the richest dietary source of MK-7. Unlike K1 from green vegetables, MK-7 has a much longer half-life (72hours vs. 1-2 hours) and is more effective at activating osteocalcin (bonehealth) and matrix Gla-protein (vascular calcification prevention).
• Bacillus subtilis spores: The spore-forming nature of B. subtilis natto means these organisms survive gastric acid and reach the intestineintact, where they can transiently colonize and produce beneficial metabolites.
For formulators, this means that a whole natto fermented extract offers a fundamentally different value proposition than isolated nattokinase. The former is a multi-mechanism cardiovascular and bone health ingredient; the latter is a targeted fibrinolytic enzyme. Both have their place, but the choice should be intentional.
Natto in Context: The Cardiovascular Ingredient Landscape
The cardiovascular supplement market is crowded with omega-3s, CoQ10, and plant sterols. Nattokinase occupies a unique position because it addresses a mechanism -fibrinolysis - that these other ingredients do not directly target. This makes it genuinely complementary rather than competitive in a stack.
Clinical trials have demonstrated that nattokinase supplementation at 2,000 FU/day over 8 weeks produced measurable reductions in fibrinogen levels and improvements in bloodviscosity. A 2022 meta-analysis published in Frontiers in Cardiovascular Medicine, covering 15 randomized controlled trials, found that nattokinase supplementation significantly reduced both systolic and diastolic bloodpressure in addition to its fibrinolytic effects.
For formulators targeting the cardiovascular category, the combination of nattokinase's unique mechanism, strong clinical backing, clean-label appeal ("fermented soybeanenzyme"), and Japan-origin premium positioning creates a compelling ingredientstory. The key is ensuring your supply chain provides vitamin K-removed nattokinase with verified fibrinolytic activity measured in standardized FU units, along with the regulatory documentation your target market requires.
Fermented Soy Tempeh: A Parallel Tradition Worth Watching
While natto uses Bacillussubtilis, another Asian fermentation tradition - tempeh - uses Rhizopusoligosporus, a filamentous fungus that produces a completely different set of bioactive compounds. Japanese researchers have been applying this organism to unexpected substrates with remarkable results.
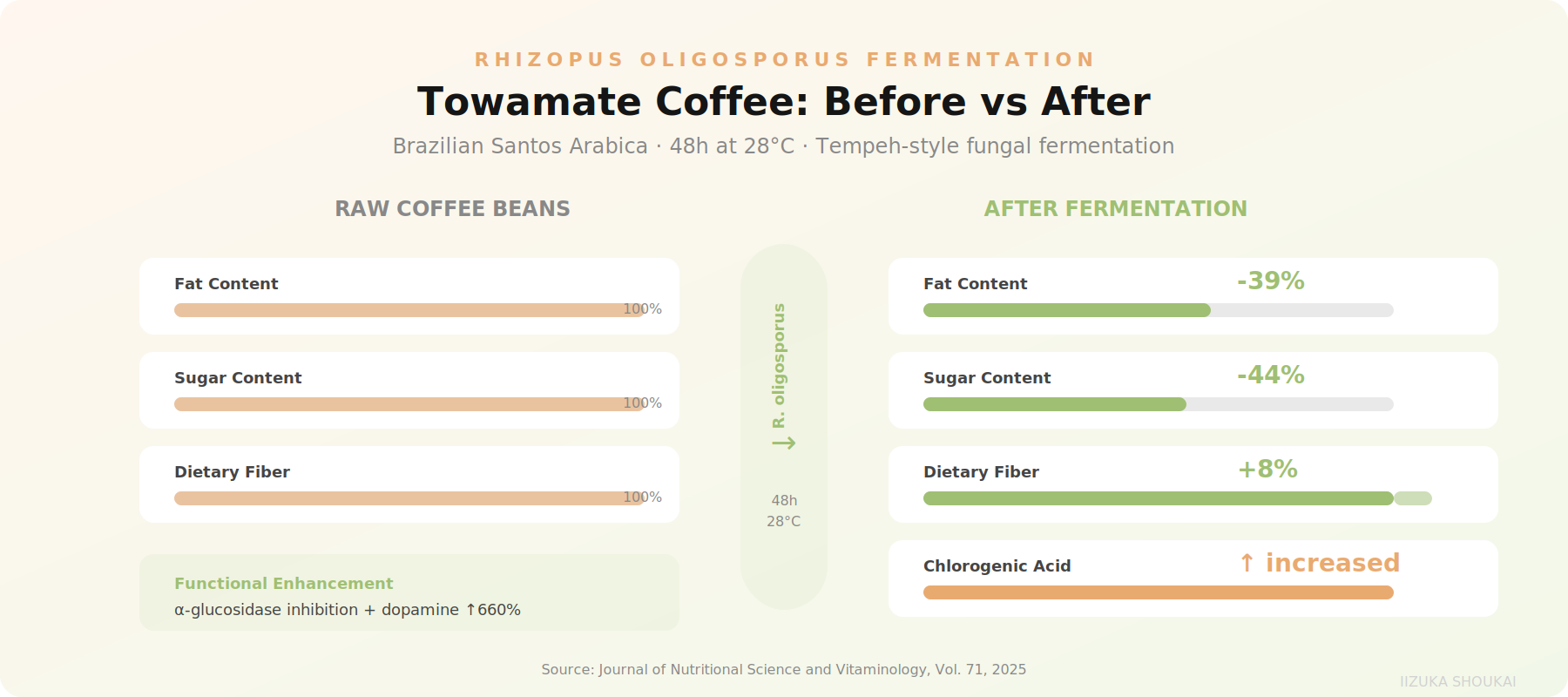
A compelling example is Towamate Coffee, developed by Towamate Co. in Hiroshima. By fermenting raw Brazilian Santos Arabica coffee beans with R. oligosporus for 48 hoursat 28 degrees C, the composition changes dramatically:
• Fat content reduced by 39%
• Sugar content reduced by 44%
• Dietary fiber increased by 8%
• Chlorogenic acid concentration increased
But the functional changes are even more interesting. Published research (Journal of Nutritional Scienceand Vitaminology, Vol. 71, 2025) demonstrates that the fermented coffee shows significantly stronger inhibition of alpha-amylase and alpha-glucosidase enzymes compared to unfermented coffee. These are the same enzymatic targets used by pharmaceutical drugs for type 2 diabetes management. The same research also showed a remarkable 660% increase in dopamine production in PC12 neuralcell models.
For the supplement industry,this represents a new category: fermentation-enhanced functional coffee ingredients for metabolic health, weight management, and cognitive function.The fermentation does not just change the coffee - it creates an entirely new functional ingredient.
Postbiotics and Lactic Acid Fermentation: Japan'sNext-Generation Gut Health Ingredients
The probiotics paradigm is shifting. While live organisms still have their place, the nutraceutical industry is rapidly recognizing that in many cases, the beneficial effects of fermented foods come not from the living bacteria themselves, but from the metabolites they produce and the cellular components they leave behind. These are postbiotics - and Japan is leading the science.
Why Postbiotics Are Winning the Formulation Battle
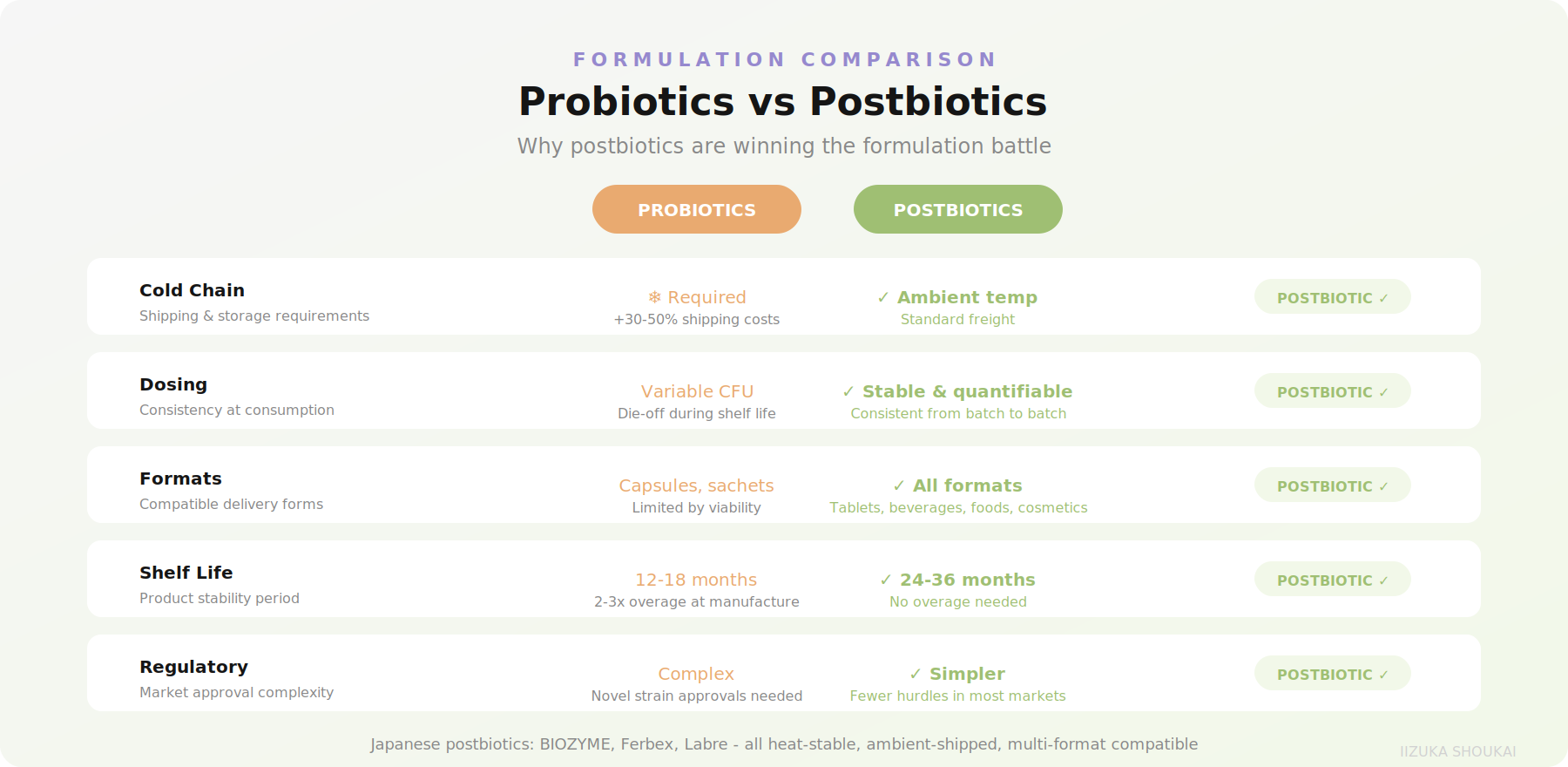
From a formulator's perspective, postbiotics solve virtually every practical problem that has plagued probiotic ingredient development:
• No cold chain: Postbiotic ingredients are heat-stable by definition. No refrigerated shipping, no cold storage, no potency loss during the product's shelf life.
• Consistent dosing: With live probiotics, CFU counts at manufacture versus at consumption can vary wildly. Postbiotic bioactive compounds are stable and quantifiable.
• Broader compatibility: Postbiotics can be incorporated into tablets, capsules, powders,functional foods, beverages, and even cosmetics without worrying about organism viability.
• Regulatory clarity: In many markets, postbiotics face fewer regulatory hurdles than live organisms, particularly for novel strains.
Barley Fermentation for Gut Health, Sleep, and Beyond
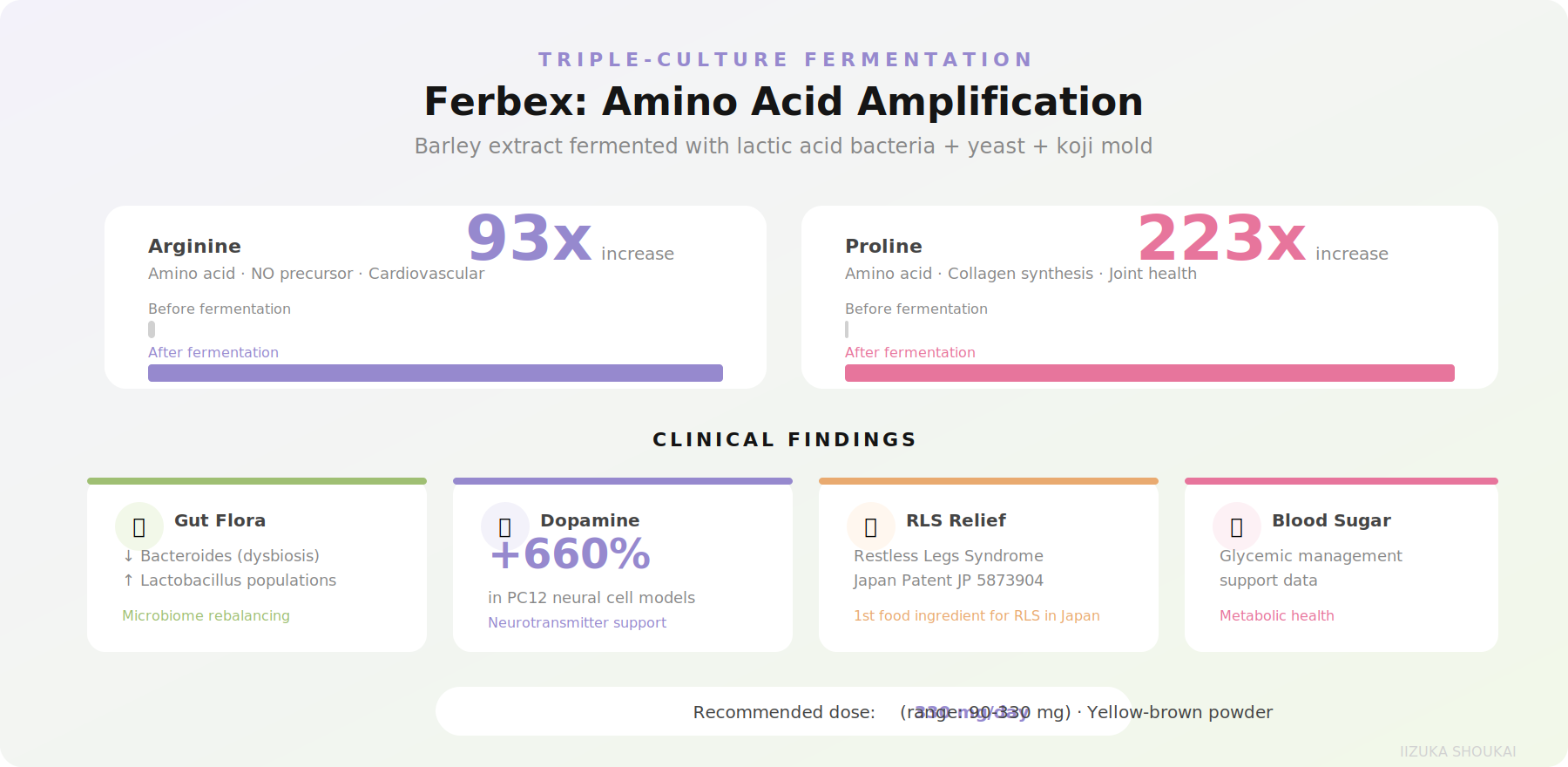
One of the most scientifically interesting postbiotic ingredients to emerge from Japan is a barley extract fermented with a triple culture of lactic acid bacteria, yeast, and koji mold. The multi-organism fermentation generates auniquely complex metabolite profile.
The fermentation dramatically amplifies the free amino acid content of the barley substrate:
• Arginine: increased 93-fold
• Proline: increased 223-fold
These are not modest changes. A 93x increase in arginine is the difference between a nutritionally insignificant source and a therapeutically relevant concentration.
Clinical research on Ferbex has revealed several unexpected benefits:
• Gut flora modulation: Decreased Bacteroides (often associated with dysbiosis) and increased Lactobacillus populations.
• Dopamine production: 660% increase in dopamine production in PC12 neural cell models - the same magnitude as observed with fermented coffee.
• Restless Legs Syndrome relief: Ferbex holds Japan Patent JP 5873904 asthe first food ingredient in Japan clinically demonstrated to relieve RLS symptoms. This represents a genuinely novel health claim for a food-derived ingredient.
• Blood sugar regulation: Supporting data for glycemic management applications.
The recommended dosage is 330 mg/day (range: 90-330 mg), making it easy to formulate into standard capsule and tablet formats. The product form is a yellow-brown powder with less than 10% moisture and a microbial limit of 3,000 CFU/g or less.
The dopamine production data for both Ferbex and Towamate Coffee point to apotentially significant connection between Japanese fermentation processes and neurotransmitter support. This is an emerging research area that could open new product positioning opportunities in the mood, focus, and cognitive health categories.
Labre: A Postbiotic Probiotic from Kyoto's Ancient Pickle Tradition
Perhaps the most elegant example of Japan's postbiotic innovation is Labre - a heat-killed preparation of Lactobacillus brevis subsp. coagulans, originally isolated from Suguki, a traditional Kyoto fermented turnip pickle with over 400 years of history.
What makes Labre distinctiveis the science behind it. The strain has been studied extensively for immune modulation, resulting in three Japanese patents and one European patent:
• JP 2051579: Immune function enhancement via activation of innate immunity.
• JP 5257363: Nano-particle, non-aggregated cell technology that maximizes immune receptor interaction.
• JP 5751219: Enhanced immunostimulant activity through proprietary processing.
• EPC 2206505: European patent covering the immune-enhancing mechanism.
The clinical mechanism centers on IFN-alpha (interferon-alpha) production boost and natural killer(NK) cell activation. This is not a vague "supports immune health" claim - this is specific, measurable activation of defined immune pathways.
From a formulation standpoint, Labre is a fully plant-origin ingredient (no animal-derived media) in tablet form, with each tablet containing approximately 3 billion nano-sized heat-killed cells. The recommended daily dose is 6 tablets (1,500 mg), delivering roughly 18 billion cells per day. Being heat-killed, the product requires no refrigeration and maintains stability across a wide range of processing conditions.
Labre has been certified by the Japan Gut Frail Conference, a recognition of its evidence-based contribution to gut-associated immune function, particularly relevant for aging populations.
The Postbiotic Advantage in Product Development Economics
Beyond the scientific and formulation advantages, postbiotics offer significant business benefits that formulators and brand owners should consider when building their product development pipeline.
Shelf life is perhaps the most impactful. Live probiotic products typically require 12-18 month shelflives with significant overage at manufacture (often 2-3x the label claim) to account for die-off. Postbiotic ingredients maintain potency for 24-36 months under standard storage conditions, with no overage requirement. This translates directly into lower ingredient costs per finished unit and reduced waste from expired inventory.
Supply chain simplicity is another factor. Postbiotic ingredients ship at ambient temperature by standard freight, rather than requiring cold chain logistics that can add 30-50% to shipping costs. For international sourcing from Japan, this is particularly relevant - your ingredients arrive by sea freight or standard air cargo, not on ice.
Finally, postbiotics open formulation formats that are simply not possible with live organisms: hot beverages, baked goods, candy and confectionery, shelf-stable shots, and cosmetic applications. Each of these represents a potential new product SKU that would be off-limits with probiotic ingredients. For brand owners looking to extend a gut health line beyond capsules and sachets, postbiotics are the enabling technology.
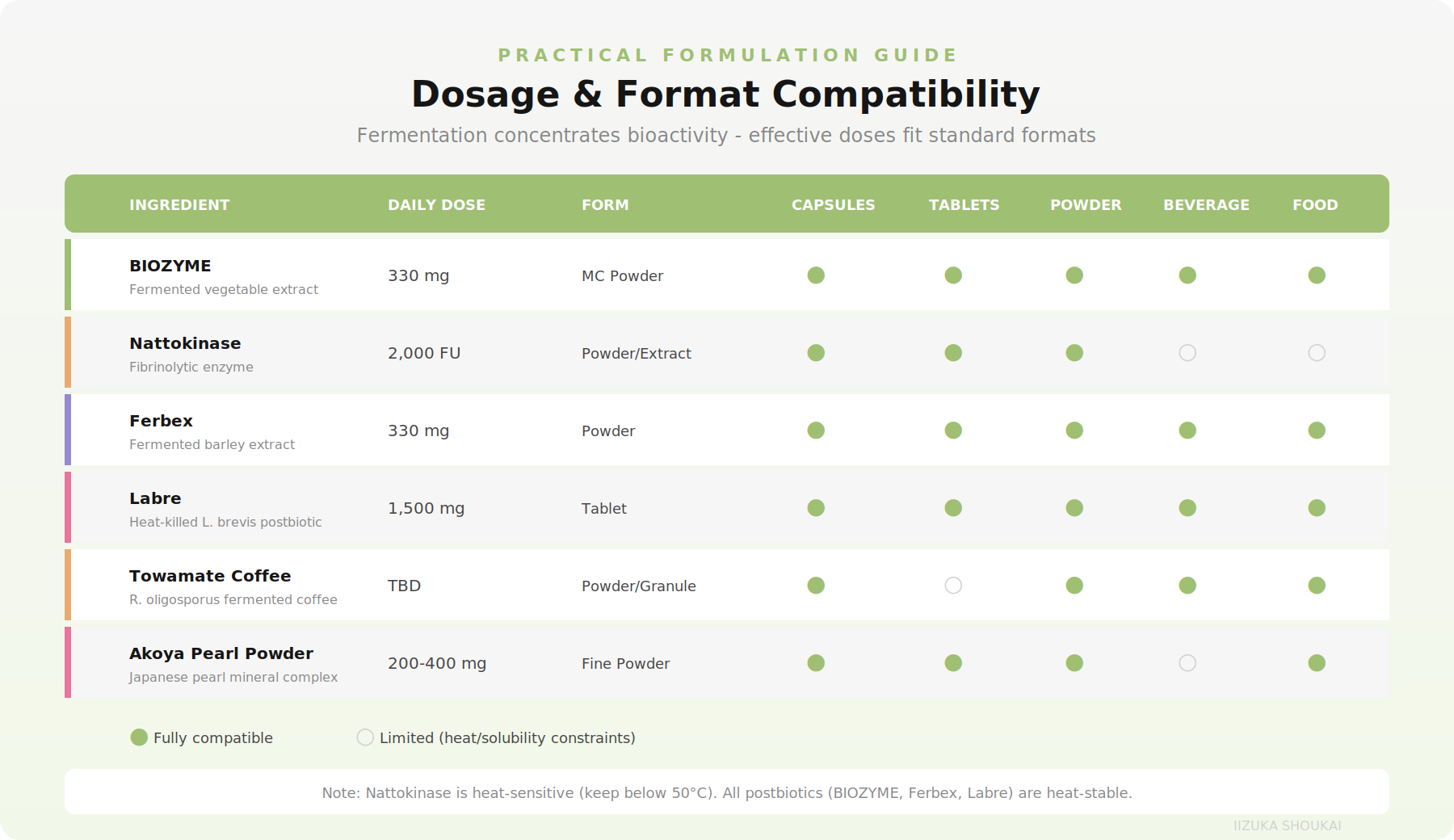
Practical Formulation Guide: Working with Japanese Fermented Ingredients
Understanding the science is only half the equation. Turning that science into commercially viable products requires practical formulation knowledge. Here is what you need to know.
Dosage and Format Compatibility
One of the significant advantages of Japanese fermented ingredients is that fermentation concentrates bioactivity, allowing effective doses to fit into standard delivery formats:
Synergistic Stacking Strategies
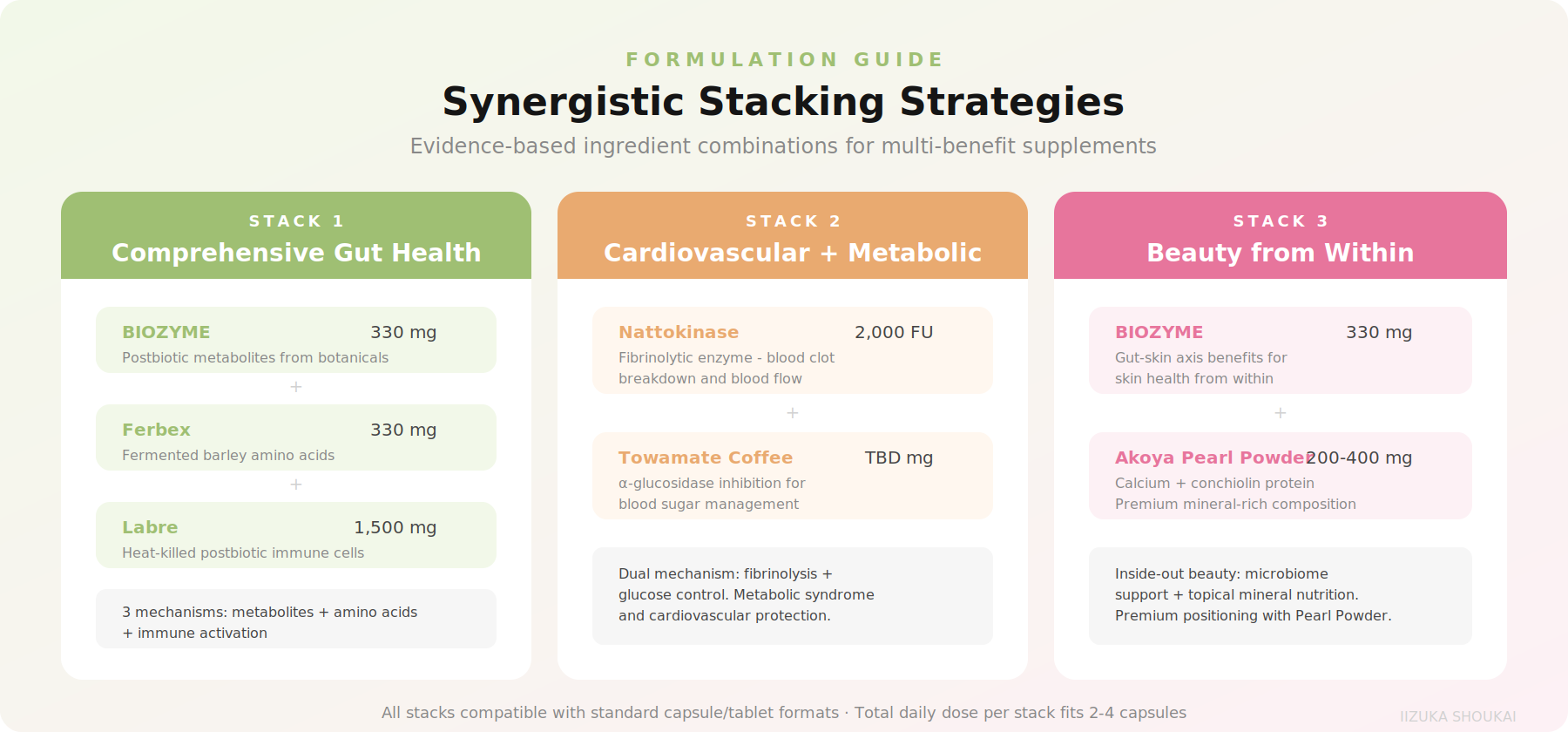
Japanese fermented ingredients work exceptionally well in combination, partly because traditional Japanese cuisine has always used multiple fermented foods together. Here are evidence-based stacking strategies:
Stack 1: Comprehensive Gut Health
BIOZYME (330 mg) + Ferbex(330 mg) + Labre (1,500 mg). This combination addresses gut health from three angles: BIOZYME provides postbiotic metabolites from botanical fermentation, Ferbex delivers fermentation-amplified amino acids for gut flora modulation, and Labre activates gut-associated immune function. Total dose fits in a reasonable dailyregimen.
Stack 2: Cardiovascular + Metabolic
Nattokinase (2,000 FU) +Towamate Coffee. Nattokinase addresses fibrinolytic and blood flow pathways, while the fermented coffee provides alpha-glucosidase inhibition for blood sugar management. This combination targets the overlapping cardiovascular-metabolicrisk profile that is increasingly common in supplement consumers.
Stack 3: Beauty from Within
BIOZYME (330 mg) + AkoyaPearl Powder. BIOZYME's gut-skin axis benefits combine with pearl powder's mineral-rich composition (calcium, conchiolin protein) for a premium beauty supplement. Pearl powder is a traditional Japanese and Chinese beauty ingredient now backed by modern composition analysis. This stack positions particularly well for the Asian beauty supplement market and for premium positioning globally.
Stability and Processing Considerations
Key technical notes for formulators working with these ingredients:
• Heat sensitivity: Nattokinase is the most heat-sensitive ingredient in this portfolio. Keep processing temperatures below 50 degrees C for nattokinase-containing products. BIOZYME, Ferbex, and Labre are all heat-stable postbiotics with no such restrictions.
• pH stability: Most Japanese fermented extracts maintain stability across a pH range of 3-8, making them compatible with both acidic beverages and neutral powder blends.
• Excipient compatibility: Dextrin is commonly used as a carrier in Japanese fermented powders (as in BIOZYME's formulation). This is well-tolerated and compatible with most standard excipient systems.
• Moisture control: Fermented powders are hygroscopic. Store below 25 degrees C with humidity control. Desiccant packs in final packaging are recommended.
• Allergen management: Soy-derived fermented ingredients (nattokinase, some fermented extracts) require allergen labeling in the EU, USA, and most other markets. The degree to which fermentation reduces allergenic proteins varies by process and should be verified with each supplier.
Regulatory Landscape by Market
Before launching fermented ingredient products, understand the regulatory framework in your target market:
• EU: Novel Food Regulation (EC 2015/2283) applies to ingredients without a history of significant consumption in the EU before 1997. Many Japanese fermented ingredients may require Novel Food authorization. Nattokinase has existing market presence in some EU member states.
• USA: Most fermented food ingredients can be marketed as dietary supplements under DSHEA,provided they meet the definition of a dietary ingredient. GRAS (Generally Recognized as Safe) status through self-GRAS determination or FDA notification strengthens market position.
• Russia and CIS: Registration as a BAD (biologically active dietary supplement) through Rospotrebnadzor or the relevant national authority. Technical documentation,certificates of analysis, and stability data are required.
• GCC/UAE: HALAL certification is essential for market access. Several Japanese fermented ingredients can obtain HALAL certification, as the fermentation organisms are plant-origin and no animal-derived media are used.
• Japan: Functional Food (Tokuho) and Food with Function Claims (FFC) pathways provide the strongest evidence framework. Products with Japanese clinical data and regulatory approval carry significant credibility in all markets.
The Japanese Advantage: Why Source Fermented Ingredients from Japan?
You might reasonably ask: if fermentation technology is available worldwide, why specifically source from Japan? The answer involves science, infrastructure, and competitive positioning.
Scientific Heritage and Process Control
Japanese fermentation science has been systematically studied and refined for over a century. The National Research Institute of Brewing (NRIB) has maintained reference strains of A. oryzae, B. subtilis natto, and other fermentation organisms since 1904. This institutional knowledge translates into a level of process control and reproducibility that newer fermentation programs simply cannot match.
When a Japanese manufacturer tells you their fermentation process takes 6 months (as with BIOZYME) or that their strain was isolated from a 400-year-old pickle tradition (as with Labre),this is not marketing storytelling. It is verifiable process documentation backed by patent filings, published research, and regulatory submissions.
Quality Infrastructure
Japan's food safety andquality infrastructure is among the most rigorous in the world. Japanese fermented ingredient manufacturers routinely provide documentation that goes far beyond what is standard in other supply markets:
• Full certificates of analysis with method validation
• Material Safety Data Sheets in multiple languages
• Stability data under accelerated and real-time conditions
• Heavy metal and pesticide residue testing to Japanese standards (whichare often stricter than EU or US requirements)
• Published clinical studies in peer-reviewed journals
• Patent documentation demonstrating novelty and prior art
Premium Positioning Opportunity
"Made in Japan"carries significant consumer cachet in the health and wellness space,particularly in Asian markets, the Middle East, and increasingly in Europe and North America. Japanese ingredients allow brands to differentiate on quality and heritage rather than competing on price against commodity suppliers.
This is not trivial. In a market where supplement brands are struggling to differentiate beyond label claims and price points, Japanese-origin fermented ingredients offer a genuinestory: centuries of tradition meeting modern clinical science, all backed by some of the world's most rigorous quality standards.
2026 Market Trends: Where Japanese Fermented Ingredients Fit
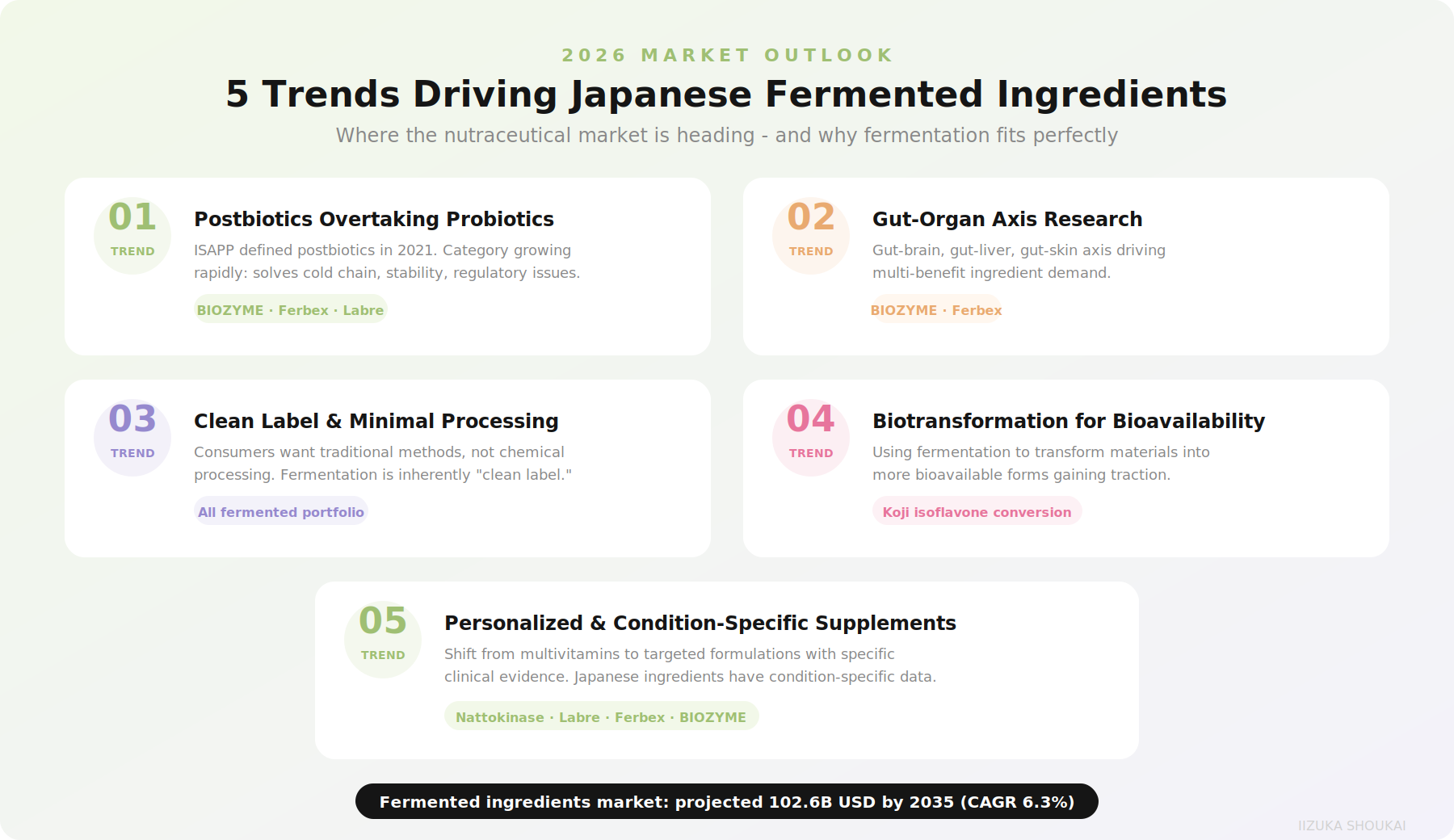
Understanding where the market is heading helps formulators make forward-looking ingredient decisions. Here are the trends most relevant to Japanese fermented ingredients:
Trend 1: Postbiotics Overtaking Probiotics
The International Scientific Association for Probiotics and Prebiotics (ISAPP) formally defined postbiotics in 2021, and the category has grown rapidly since. Postbiotics solve the coldchain, stability, and dosing consistency problems that have limited probiotic ingredient adoption. Japanese manufacturers were early to this space -ingredients like BIOZYME, Ferbex, and Labre are all postbiotic by design,reflecting Japan's long understanding that fermentation metabolites, not just live organisms, drive health benefits.
Trend 2: Gut-Organ Axis Research
The gut-brain axis is well established. The gut-liver axis is gaining recognition. The gut-skin axis is driving the beauty-from-within category. Japanese fermented ingredients are uniquely positioned here because traditional Japanese fermentation was always about whole-body health, not isolated mechanisms. BIOZYME's clinical data spanning gut, liver, and skin endpoints is a perfect example of this multi-axisapproach.
Trend 3: Clean Label and Minimal Processing
"Clean label" increasingly means more than just recognizable ingredient names. Consumers and regulators want to see minimal chemical processing and traditional production methods. Fermentation is inherently clean-label - it is a biological transformation,not a chemical extraction. Japanese fermented ingredients that can trace their production line age to centuries-old traditions have a particularly compelling clean-label story.
Trend 4: Biotransformation for Enhanced Bioavailability
The concept of using fermentation to transform raw materials into more bioavailable forms is gaining traction across the nutraceutical industry. This is exactly what Japanese fermentation does: koji enzymes converting isoflavone glycosides to aglycones, nattobacteria transforming soy proteins into bioactive peptides, lactic acidfermentation amplifying amino acid content by orders of magnitude. The industry is catching up to what Japanese food science has known for generations.
Trend 5: Personalized and Condition-Specific Supplements
The shift fromone-size-fits-all multivitamins to condition-specific formulations favors specialized ingredients with targeted clinical evidence. Japanese fermented ingredients, with their specific clinical data (Ferbex for RLS, nattokinase forfibrinolysis, Labre for NK cell activation), fit perfectly into this trend.
Getting Started: From Interest to Formulation
If this article has opened your eyes to the potential of Japanese fermented ingredients, the natural next question is: what now?
Here is a practical roadmap:
• Step 1 - Define your target application: Are you developing for gut health,cardiovascular, beauty, metabolic, immune, or cognitive categories? Japanese fermented ingredients span all of these, but your starting point determines which ingredients to evaluate first.
• Step 2 - Request samples and documentation: Technical evaluation requires physical samples for your R&D team, plus full documentation packages including specifications, COAs, MSDS, clinical study summaries, and regulatory status by market. A serious supplier provides all of this as a standard part of the evaluation process.
• Step 3 - Assess regulatory pathway: Based on your target market, determine what regulatory submissions or notifications are required. Japanese ingredients with existing clinical data and patent protection significantly streamline this process.
• Step 4 - Prototype and test: Develop prototype formulations, assessstability, and conduct any required safety or efficacy testing for your specific product claims.
• Step 5 - Scale and launch: Work with your ingredient supplier to secure supply agreements, establish quality specifications, and align production timelines.
Ready to Explore Japanese Fermented Ingredients?
Iizuka Shoukai is a Japan-based B2B trading company specializing in premium Japanese functional ingredients for the nutraceutical, cosmetic, and functional food industries. We work directly with Japanese manufacturers to provide international brands and formulators with access to clinically validated fermented ingredients, complete technical documentation, and regulatory support for your target markets.
What we can provide: Technical samples of all ingredients discussed in this article, full specification packages,certificates of analysis, MSDS documentation, clinical study summaries,regulatory guidance for EU, USA, Russia/CIS, GCC, and other markets.
Minimum order quantities start from 1 kg for most ingredients, with competitive pricing at production volumes.
Contact us: iizuka.shoukai@gmail.com |iizuka-shoukai.com
Request a free sample kit of Japanese fermented ingredients for your R&D evaluation. Include your target application, volume requirements, and regulatory market in your inquiry for the fastest response.
---
About the author: Anna Iizuka is Executive Director of Iizuka Shoukai, LLC, working at the intersection of Japanese ingredient science and international supplement markets. For technical inquiries about any ingredient discussed in this article, contact iizuka.shoukai@gmail.com.

